Hepatitis C Rapid Test Kit (40 pcs/box)
Hepatitis C Rapid Test Kit (40 pcs/box)
CarbonMedical
Couldn't load pickup availability
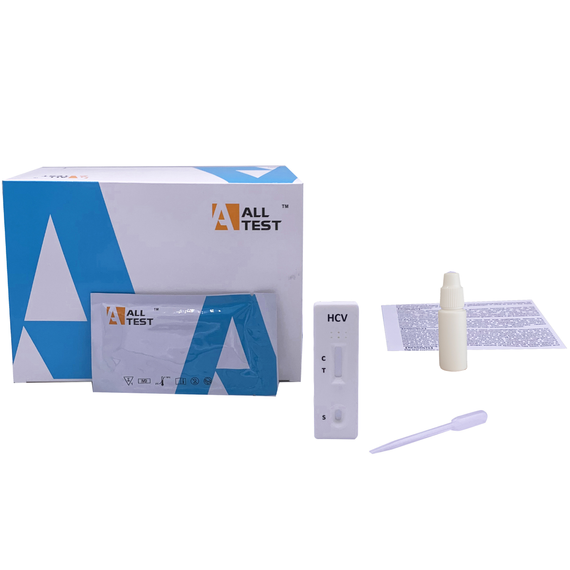
Hepatitis C Rapid Test Kit (40 pcs/box)
The Hepatitis C Rapid Test is a user-friendly, painless, and fast solution for detecting the Hepatitis C Virus (HCV). It provides reliable results from a blood sample within minutes, enabling early detection and timely treatment. This test is ideal for individuals who have been exposed to risk factors such as tattoos, piercings, blood transfusions, or intravenous drug use.
HCV is a liver-targeting virus that can cause both acute and chronic hepatitis. It is primarily transmitted through blood, for example via unsterile medical tools, blood transfusions, or intravenous drug use. Globally, more than 70 million people are estimated to live with chronic Hepatitis C infection, which may lead to serious complications like liver cirrhosis or liver cancer. Early diagnosis is essential, as modern antiviral therapies offer cure rates of up to 95%. Since the infection is often asymptomatic, regular screening is especially important for high-risk groups.
The HCV Rapid Test is a chromatographic immunoassay for the qualitative detection of HCV antibodies in human whole blood, serum, or plasma. It is intended for professional in vitro diagnostic use only and provides a quick and reliable tool for healthcare professionals to screen for HCV infection.
Specifications
Product Name: HCV Rapid Test Cassette
Sample Type: Whole blood / Serum / Plasma
Format: Cassette
Packaging: 40 tests/box
Manufacturer: Hangzhou Alltest Biotech Co., Ltd., 550 Yinhai Street, Hangzhou Economic and Technologic Development Area, 310018 Zhejiang, China, Tel: +86-571-56267891, Email: info@alltests.com.cn
EC REP: MedNet GmbH, Hansestraße 91, 48165 Münster, Germany, Tel: +49 251 32266-0, Email: info@medneteurope.com
Expiry Date: 2026.09
Kit Contents
-
40 test cassettes
-
40 disposable droppers
-
2 buffer solutions
-
1 instruction manual in English
Test Procedure
Read the instructions carefully before use. Perform sampling as described in the manual. Allow all components (reagents, samples, and controls) to reach room temperature (15°C-30°C) before use.
1. Preparation
Before opening, allow the sealed pouch to reach room temperature. Remove the test cassette from the foil pouch and use it as soon as possible. Perform the test within one hour of opening the pouch for optimal results.
2. Sample Application
-
2.A Serum/Plasma:
Hold the dropper vertically and dispense 1 drop of serum/plasma (~25 μL) into the sample well (S), then add 2 drops of buffer (~80 μL). Start the timer. -
2.B Venous Whole Blood:
Hold the dropper vertically and dispense 2 drops (~50 μL) of venous whole blood into the sample well (S), then add 2 drops of buffer (~80 μL). Start the timer. -
2.C Fingerstick Whole Blood:
Fill the capillary tube with fingerstick blood and dispense approximately 2 drops (~50 μL) into the sample well (S), followed by 2 drops of buffer (~80 μL). Start the timer.
Note: Avoid air bubbles in the sample well. Do not apply buffer directly to the result area.
3. Read the result after 10 minutes.
Do not interpret the result after 20 minutes.
Note: Do not use the buffer solution more than 6 months after opening.
Interpretation of Results
Positive
Two colored lines appear - one in the control region (C) and one in the test region (T). The presence of a T-line, regardless of intensity, indicates a positive result for HCV antibodies.
Negative
Only one colored line appears in the control region (C); no line in the test region (T).
Invalid
No line appears in the control region (C). The test is invalid due to insufficient sample, incorrect procedure, or expired test. Repeat the test using a new cassette. If the issue persists, contact your distributor.
Test Performance
When compared to CE-marked EIA or CMIA reference methods, the HCV Rapid Test Cassette (whole blood/serum/plasma) demonstrated:
-
Relative Sensitivity: 100%
-
Relative Specificity: 100%
Legal Notices
Professional Use
This product is an in vitro diagnostic (IVD) and intended exclusively for professional use by medical personnel. Use for self-testing is not permitted. The manufacturer/distributor accepts no liability for damages resulting from improper or unauthorized use.
Right of Withdrawal
In accordance with § 312g (2) No. 3 of the German Civil Code (BGB), there is no right of withdrawal for sealed goods which, for reasons of health protection or hygiene, are not suitable for return if their seal has been removed after delivery.
Disclaimer
The seller assumes no responsibility for compliance with legal regulations within the buyer’s procurement process. The seller’s responsibility is limited solely to the delivery of the ordered goods and the provision of information regarding their proper use.

